HCOOCH CH2 H2O: What it Really Means Simple Guide
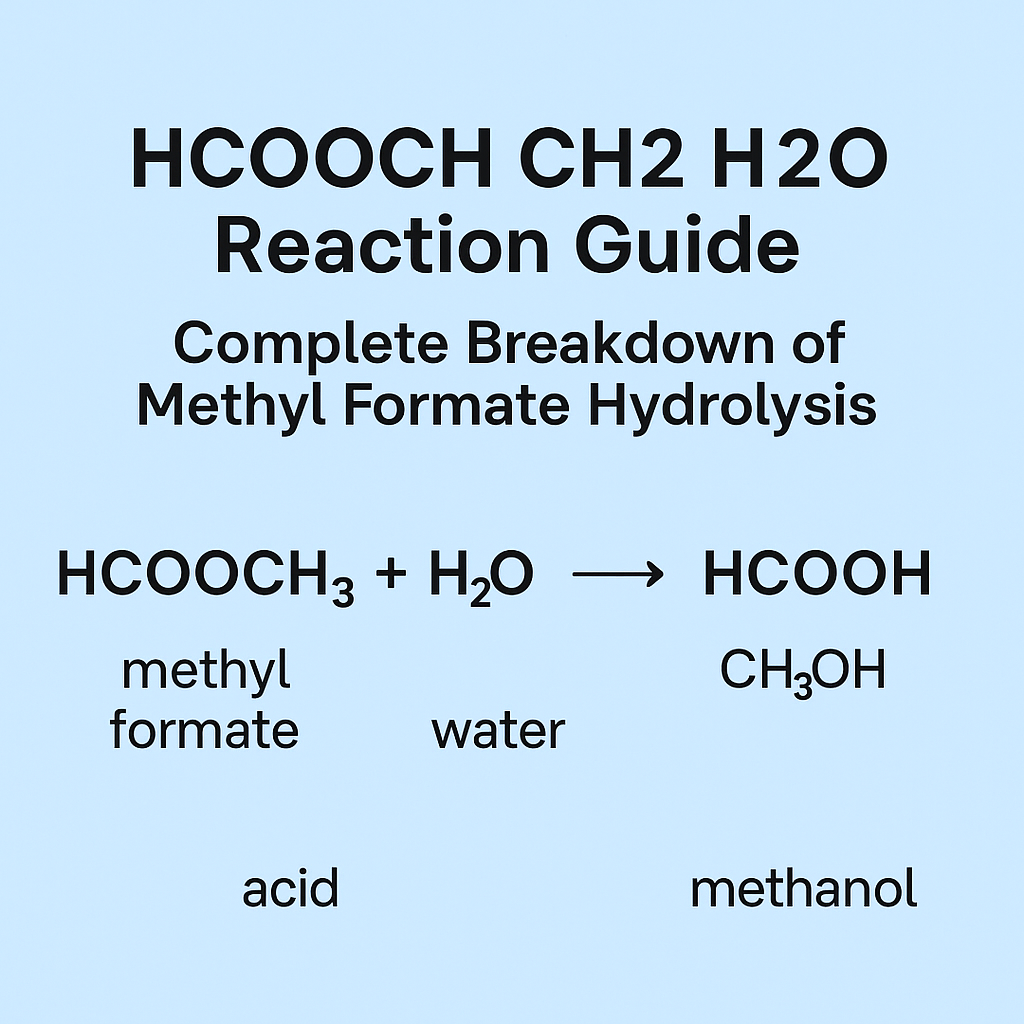
Many websites show the string “HCOOCH CH2 H2O.” It looks like a chemical formula, but it is not a proper name for one single molecule. In most cases, people use this string when they talk about the hydrolysis (reaction with water) of the ester called methyl formate. In simple words: methyl formate + water → formic acid + methanol Aurö
This reaction belongs to a common group of changes in organic chemistry called “ester hydrolysis.” It is important in classrooms and also in factories. It helps make useful products in a way that can be safe and efficient when done with the right setup.
First, fix the confusing name
The string “HCOOCH CH2 H2O” is confusing for beginners. The part “CH2” here is not correct for this reaction. The correct ester is methyl formate, which has a methyl group CH3, not CH2 on its own. So, when you see “HCOOCH CH2 H2O,” think of this instead:
HCOOCH3 (methyl formate) + H2O (water) → HCOOH (formic acid) + CH3OH (methanol)
That is the safe, standard way to read the topic.
The key players (reactants and products)
Methyl formate (HCOOCH3)
-
A simple organic liquid.
-
Has a pleasant smell.
-
Evaporates easily because it boils at a low temperature.
-
It is an ester, which means it can break apart in water under the right conditions.
Water (H2O)
-
The most common solvent in chemistry.
-
In this reaction, it is not only a solvent. It also takes part directly and helps cut the ester into two new pieces.
Formic acid (HCOOH)
-
The simplest carboxylic acid.
-
Acidic and can be corrosive, so we handle it with care.
-
Used in many industries.
Methanol (CH3OH)
-
A simple alcohol.
-
Useful as a solvent and for making other chemicals.
-
Flammable and toxic if swallowed, so it needs careful handling.
The balanced reaction (in words)
One molecule of methyl formate and one molecule of water react together. The products are one molecule of formic acid and one molecule of methanol. In symbols (written plainly):
HCOOCH3 + H2O → HCOOH + CH3OH
This is a balanced reaction. The number of each type of atom is the same on both sides.
How the reaction happens (mechanism) in easy terms
There are two common ways for an ester to break apart in water.
Acid-catalyzed hydrolysis
-
An acid in the mixture helps “activate” the ester by adding a proton to it. This makes the key carbon more ready to react.
-
Water attacks that carbon. A temporary structure forms.
-
The structure then breaks into two products: formic acid and methanol.
-
The acid you used to help the reaction comes back in the end (that is why we call it a catalyst).
Base-catalyzed hydrolysis (saponification)
-
A base (like OH–) attacks the key carbon on the ester.
-
The ester opens up and forms a salt of formic acid plus methanol.
-
In this version, the base is not simply “given back” at the end in the same way as the acid case. Also, the free acid is not formed until you add an extra acid step later.
Both paths are common in teaching. For methyl formate, the acid path is very typical when people want free formic acid as a product right away.
What affects the reaction (conditions that matter)
Some settings make the reaction faster and more complete. For real-world work, you want speed, control, and good yield. The table below lists the most important factors and what they do.
Key factors that change speed and yield
| Factor | What it is | What it does |
|---|---|---|
| Catalyst (acid or base) | A small amount of acid (H+) or base (OH–) added | Speeds up the reaction a lot |
| Temperature | How hot the mixture is | Higher temperature usually makes it faster |
| Water amount | The ratio of water to methyl formate | Extra water helps push the reaction toward products |
| Pressure (in closed systems) | Common in factory setups | Helps keep liquids from boiling off; improves control |
| Product removal | Taking out methanol or water during/after reaction | Helps drive the reaction forward and improve yield |
Simple rule: more water, some heat, and a helpful catalyst make the process better. Removing the methanol as it forms can also shift the balance toward more product.
Basic properties that guide safe work
We need to know how these substances behave. That helps us store, move, heat, and separate them safely and efficiently.
Simple property guide (at normal pressure)
| Substance | Short notes | Why it matters |
|---|---|---|
| Methyl formate (HCOOCH3) | Volatile, low boiling point, flammable | Needs closed equipment; watch sparks and heat |
| Water (H2O) | Safe, common solvent | Drives hydrolysis; easy to handle |
| Formic acid (HCOOH) | Acidic, corrosive | Use proper materials and protective gear |
| Methanol (CH3OH) | Volatile, flammable, toxic if swallowed | Ventilation, no flames, careful storage |
These notes are enough for a quick safety view. In a lab or plant, you would check a safety data sheet for full details.
Why this reaction is useful
This reaction is not just a textbook item. It matters in real life.
-
It is a clear example of ester hydrolysis, which students study early in organic chemistry.
-
It is used in industry to make formic acid on a large scale.
-
It gives methanol as a useful partner product.
-
It fits well with green chemistry ideas because water is a clean reagent, and conditions can be moderate.
Where the products go
Formic acid and methanol have many uses. They move into different parts of the chemical world.
-
Formic acid is used in leather processing, textiles, rubber, and animal feed preservation. It also helps in some clean-energy systems as a hydrogen carrier.
-
Methanol is used as a fuel component, as a solvent, and as a starting point to make other key chemicals (like formaldehyde and acetic acid).
Simple overview of an industrial setup
A factory does not usually run this reaction in a small flask. It uses equipment that keeps things safe and efficient.
-
Feed tanks store methyl formate and water.
-
A pump sends them into a heated reactor. The reactor may run under pressure to keep everything liquid and steady.
-
After reaction, the mixture goes to separators and distillation units. These units take out methanol, leftover ester, and water. Formic acid is collected at the right purity.
-
Unreacted methyl formate can go back (recycle) to the reactor to reduce waste and improve overall yield.
The big ideas are control, separation, and recycle. With these, the process can run day and night with steady quality.
Uses of HCOOCH CH2 H2O
Below are exactly two lists, as requested. The first list covers common uses of formic acid. The second list is a safety checklist for this reaction system.
Common uses HCOOCH CH2 H2O
-
Leather tanning and textile treatment
-
Preserving animal feed and silage
-
Cleaning and pickling metals
-
Rubber and polymer processing
-
Making dyes, drugs, and other fine chemicals
-
As a liquid carrier of hydrogen in some energy systems
-
pH control in various industrial steps
Safety checklist for this reaction
-
Keep away from flames and sparks (methyl formate and methanol are flammable).
-
Use closed equipment or good ventilation to manage vapors.
-
Wear gloves and goggles when handling formic acid.
-
Ground and bond containers to prevent static discharge.
-
Control temperature to avoid pressure jumps and hot spots.
-
Store methanol and methyl formate in proper, labeled containers.
-
Neutralize acidic waste before disposal; follow local rules.
Simple tips for better yield and cleaner process
-
Use a small amount of acid catalyst if you want the free acid (formic acid) directly.
-
Use extra water to push the balance toward products.
-
If possible, remove methanol as it forms. This helps “pull” the reaction forward.
-
Run at a steady, moderate temperature. Do not guess; use a setpoint your equipment can hold well.
-
If you get a lot of unreacted ester, try recycling it back to the reactor feed.
These steps help you get more formic acid per unit of time and reduce waste.
Common mistakes and how to avoid them
-
Mistake: Treating “HCOOCH CH2 H2O” as one single compound.
Fix: Remember it is shorthand for methyl formate plus water in a hydrolysis context. -
Mistake: Thinking hydrolysis always goes to 100% by itself.
Fix: Use enough water, use a catalyst, and remove products to drive the reaction forward. -
Mistake: Mixing up hydrolysis with esterification (the reverse).
Fix: Hydrolysis breaks the ester into acid + alcohol. Esterification builds the ester from acid + alcohol. Keep water high to favor hydrolysis. -
Mistake: Ignoring safety.
Fix: Plan for flammable vapors (methyl formate, methanol) and corrosive acid (formic acid). Use proper gear and ventilation.
How this fits with green chemistry
HCOOCH CH2 H2O reaction is a nice match with green chemistry ideas.
-
Water is a safer solvent than many organic liquids.
-
The products are useful and can be recovered and reused.
-
The reaction can run at moderate heat.
-
With good design, waste can be low, and energy use can be smart (for example, using heat exchange and recycling streams).
All of this reduces impact on air, water, and energy use.
A closer look at learning value
Teachers like this HCOOCH CH2 H2O for many reasons:
-
It shows how catalysts work without getting lost in complex math.
-
It shows that “equilibrium” is real: not every reaction goes to full completion.
-
It lets students connect lab chemistry to real production.
-
It trains safe thinking: identify hazards, pick the right gear, control a process.
Students who understand this reaction learn skills they will use again and again.
fAQs
Is “HCOOCH CH2 H2O” a correct formula?
No. It is not a standard name. Think of it as a rough way some sites use to point to methyl formate plus water in a hydrolysis topic.
What are the real products of HCOOCH CH2 H2O?
Formic acid and methanol.
Do I need a catalyst?
A small amount of acid often helps a lot. It makes the reaction faster and more reliable.
Can I push the reaction to make more product?
Yes. Use extra water, keep the temperature steady, and remove methanol or water as needed to shift the balance.
HCOOCH CH2 H2O is safe?
It can be safe if you follow rules. Avoid flames, use ventilation, protect your skin and eyes, and manage waste correctly.
Conclusion
HCOOCH CH2 H2O is a simple reaction with big value. It shows how clear thinking and good setup can turn a basic lab idea into a strong, repeatable process. When you see the confusing string “HCOOCH CH2 H2O,” remember the real message: methyl formate plus water gives formic acid and methanol. Keep the language simple, keep the steps clear, and the chemistry makes sense.